Neurodevelopmental Deficits Prediction
Introduction: With the continuing high incidence of preterm births (about 380,000 in 2018) and improving survival rates (exceeding 90%) in the United States, the prevalence of disabled survivors of prematurity has increased dramatically. Particularly, survivors following very premature birth (i.e., 32 weeks gestational age) are at high risk for neurodevelopmental impairments, thereby increasing their risk for poor educational, health, and social outcomes. Unfortunately, it may take 2 to 5 years from birth to identify such high-risk infants. Currently, neonatologists and neurologists have limited tools available for counseling parents of very premature infants on their childs likely outcomes. Early, accurate identification, soon after birth, could pave the way for domain-specific, intensive early neuroprotective therapies during a critical window for optimal neuroplasticity.
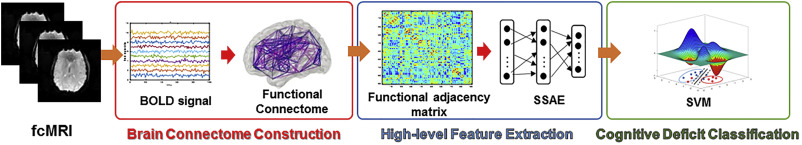
Clinicians typically look for brain abnormalities in a few key brain regions. However, our pilot studies have shown that outcomes are associated with voxels and sub-regions that are not typically recognized by experts. Additionally, up to 80% of very preterm infants have no abnormalities reported on anatomical MRI at term, yet nearly 30% of these infants still develop adverse outcomes. Clearly, a critical gap in knowledge and methodology remains, and there is a need to identify novel and effective early prognostic MRI markers using high-dimensional 1) quantitative anatomical features, characterizing spatial appearances and spectral properties of fine-scale, pre-defined structural parcellations/sub-regions through signal intensity distribution, morphology, and texture; and 2) quantitative mapping of the structural and functional connections within and among whole-brain parcellations. We have previously implemented traditional multivariable statistical analyses and machine learning to predict neurodevelopmental deficits and identify several prognostic MRI anatomical and connectome markers, but these approaches are inadequate to properly model the high-dimensional data. Alternatively, deep learning is well-suited for extracting biologically meaningful and discriminative information from high-dimensional MRI data that are optimal for outcome prediction at an individual level. Studies have shown that application of deep learning to brain MRI data can detect anatomical abnormalities, disrupted connectivity, and discover discriminative markers that best predict outcomes.
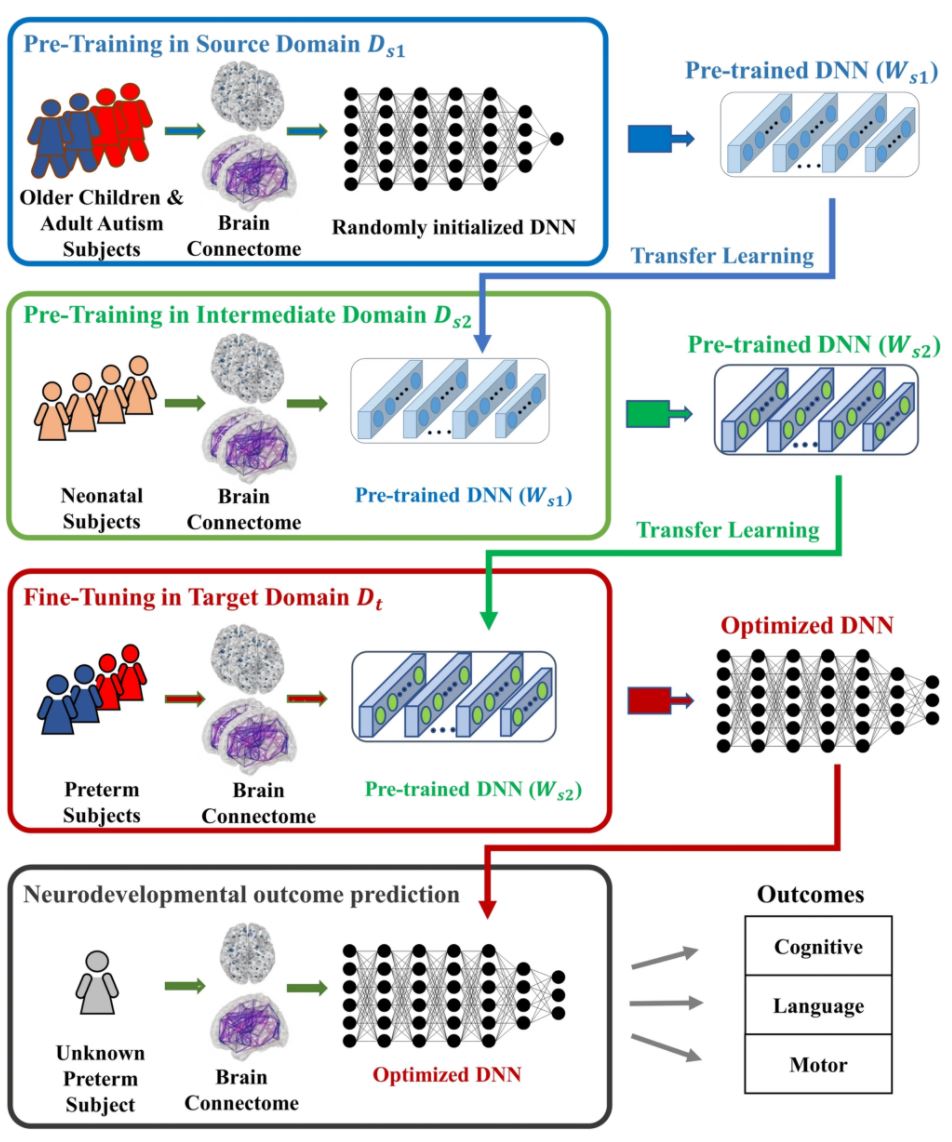
Deep learning has achieved breakthroughs in applications with large sample sizes. However, when facing high-dimensional, low-sample-size annotated neuroimaging datasets, deep learning suffers from insufficient training data. Transfer learning represents an important advance to solve this fundamental problem. It focuses on storing knowledge gained from solving problems in one data-rich domain (source) and applying it to a related new problem in another domain (target).
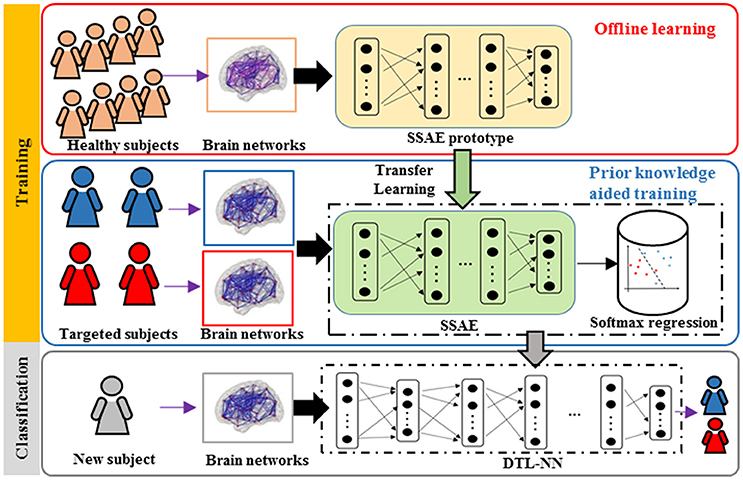
Our group has previously proposed a Stacked Sparse Autoencoder based neural network framework for early prediction of cognitive deficits, one of common neurodevelopmental deficits, in very preterm infants based on functional connectome data. (He, Li et al. 2018) In this work, we applied a transfer learning approach to mitigate the insufficient neuroimaging data issue. Our results offer a proof of concept for the use of deep learning models over conventional models to enhance diagnosis or prediction of cognitive deficits in very preterm infants.
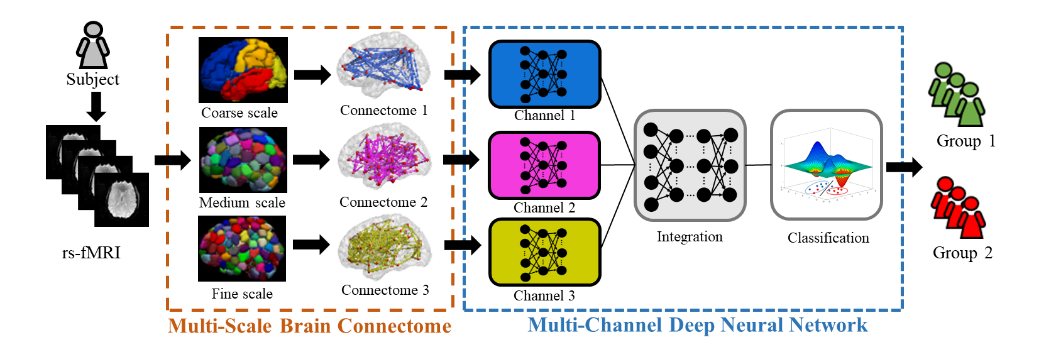
We also developed a transfer learning-enhanced convolutional neural networks using structural brain connectome data to predict cognitive deficits in very preterm infants. (Chen, Li et al. 2020) The proposed model achieved improved performance by integrating multiple technique advances, including the convolution on adjacency matrix, transfer learning strategy, and data balance and augmentation approach. The promising results suggest that deep learning models and structural connectome may facilitate early prediction of later neurodevelopmental outcomes in very preterm infants at term-equivalent age.
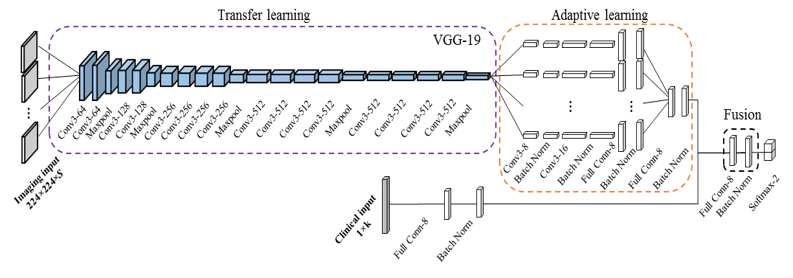
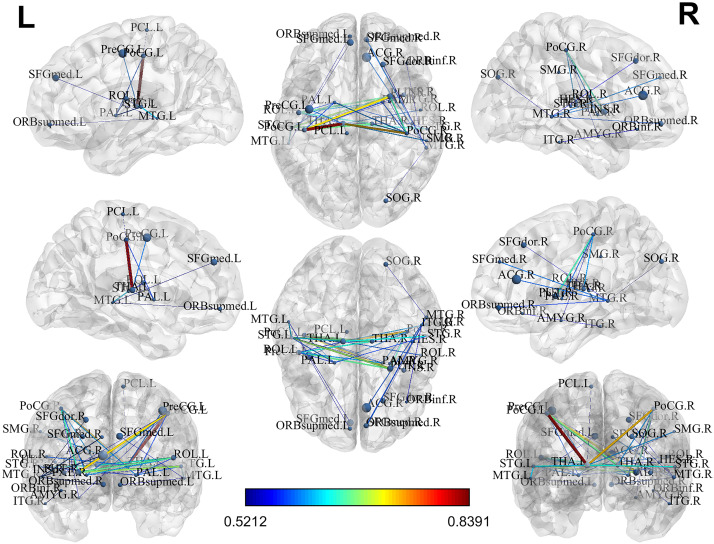
Recently, we extended our research focus to multiple neurodevelopmental deficits, including cognitive, language and motor deficits. To jointly predict multiple neurodevelopmental deficits in very preterm infants, we developed a multi-task, multi-stage deep transfer learning framework using the fusion of brain connectome and clinical data. (He, Li et al. 2020) Intriguingly, we found that the knowledge gained from classifying Autism Spectrum Disorder (ASD) patients versus typically developing controls in children and adults can be transferred to predict neurodevelopmental deficits in infants. The proposed model not only outperformed several peer methods, but also identified multiple brain regions, including thalamus, middle temporal gyrus, inferior frontal gyrus, fusiform gyrus, and paracentral gyrus, among others, which serve important functions for language, sensory, motor, object vision, and cognitive function.
Reference:
He L, Li H, Holland SK, Yuan W, Altaye M, & Parikh NA. (2018). Early prediction of cognitive deficits in very preterm infants using functional connectome data in an artificial neural network framework. Neuroimage Clin, 18, 290-297. PMID: 29876249; PMCID: PMC5987842. Pubmed Journal
He L, Li H, Wang J, Chen M, Gozdas, E, Dillman JR, Parikh NA, (2020). A multi-task, multi-stage deep transfer learning model for early prediction of neurodevelopment in very preterm infants. Sci Rep 10, 15072 (2020). PMCID: PMC7492237 PMC Journal